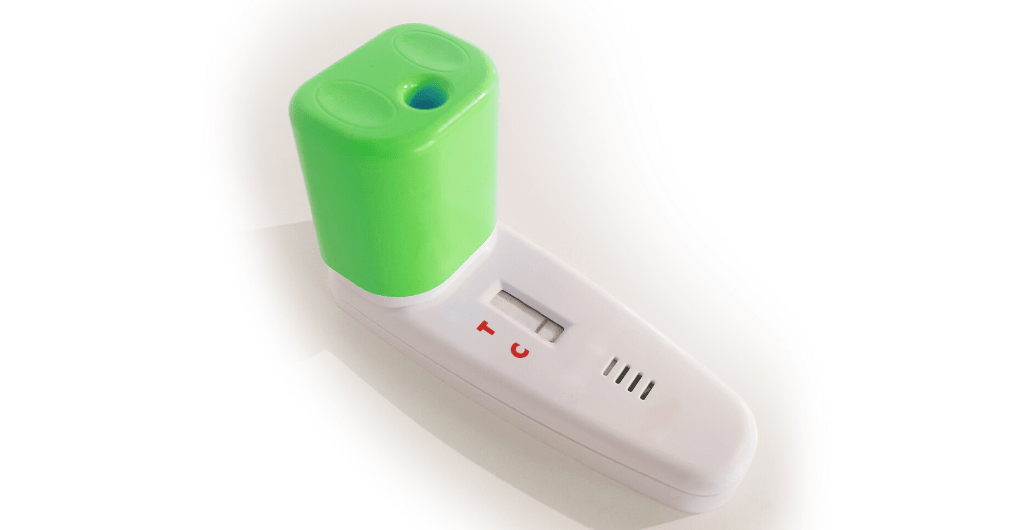
New rapid diagnostics sputum based kit for home use expected to be completed in 3-4 months
BATM (LSE: BVC; TASE: BVC), a leading provider of real-time technologies for networking solutions and medical laboratory systems, announces that it has entered into a collaboration with Novamed Ltd, an Israeli life sciences company operating in the in-vitro rapid diagnostics market, for the joint development and marketing of a rapid testing kit for home use for diagnosing COVID-19.
The new kit, which allows people to test a sputum sample at home and receive results within a few minutes, is for the identification of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19). The parties anticipate that the development of the kit will be completed within 3-4 months after which, following the receipt of CE certification, the parties expect the kits to be available for sale first in Israel and then globally.
In addition, further to the Group’s announcement of 27 February 2020, BATM is pleased to report that it is starting to ship its new diagnostic kit for COVID-19 that was developed by Adaltis for use by medical facilities. The initial customers are European, with delivery to Italy being the current focus. The Group is preparing to ramp up production by the end of next week to meet existing orders and anticipated demand. All clinical testing to date has demonstrated the excellent performance of this kit, which provides results in under one hour on COVID-19 and other variants of coronavirus using real-time PCR (polymerase chain reaction), a molecular biology diagnostic lab technique.
Dr Zvi Marom, Chief Executive Officer of BATM, said: “This is another step forward in our strategy to provide effective solutions for tackling infectious diseases. The COVID-19 outbreak has demonstrated the huge challenges that are posed by such diseases and the importance of having a comprehensive response covering rapid diagnosis and treatment.
“Alongside our COVID-19 diagnostic kit for use in medical facilities, we recognised the necessity for an easy-to-use and reliable test that can be done at home to help reduce the pressure on the already-congested hospitals and minimise patient exposure.
“We chose to partner with Novamed thanks to their many years of experience in developing excellent at-home diagnostic products. We believe that the combination of the capabilities of BATM and Novamed will result in a uniquely effective solution to address the current pandemic as well as other outbreaks in the future.”








