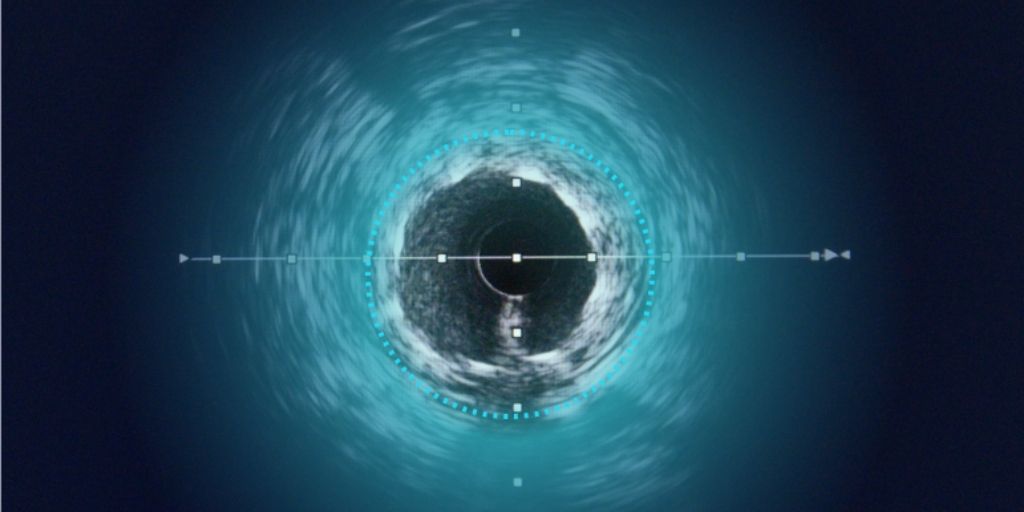
 Dyad Medical, Inc., the developer of the cloud-based AI technology for cardiac image analysis called LibbyTM, announced that the U.S. Food and Drug Administration (FDA) has cleared LibbyTM IAAA through the 510(k) pathway. LibbyTM IAAA offers image analysis used for viewing and quantifying Intravascular Optical Coherence Tomography (IVOCT) images. The clearance provides healthcare professionals another important tool to automate intravascular investigation, or the ability to see the inside of blood vessels.“Dyad Medical is proud to offer hospital systems, imaging centers, and researchers a tool to conduct cardiac image analysis in a fraction of the time,” said Dr. Ronny Shalev, CEO and Co-Founder of Dyad Medical. “This new milestone indicates the FDA’s trust in Dyad Medical’s work and enables broader adoption of imaging AI as an integral part of value-based care.”Coronary artery disease, the most common type of heart disease, is the leading cause of death, disability, and escalating healthcare costs worldwide. It is frequently treated by inserting metal stents in the hearts’ arteries. The stents help hold open the artery, resolving obstructions and blockages that impede coronary blood flow. While drug-eluting stents hinder restenosis, or future blockages, there is room to improve existing treatment options. To optimize stent design, sensitive in vivo, or within the heart, assessments are needed for preclinical and clinical evaluations. IVOCT is the only imaging method with the resolution and contrast able to accomplish this.
Dyad Medical, Inc., the developer of the cloud-based AI technology for cardiac image analysis called LibbyTM, announced that the U.S. Food and Drug Administration (FDA) has cleared LibbyTM IAAA through the 510(k) pathway. LibbyTM IAAA offers image analysis used for viewing and quantifying Intravascular Optical Coherence Tomography (IVOCT) images. The clearance provides healthcare professionals another important tool to automate intravascular investigation, or the ability to see the inside of blood vessels.“Dyad Medical is proud to offer hospital systems, imaging centers, and researchers a tool to conduct cardiac image analysis in a fraction of the time,” said Dr. Ronny Shalev, CEO and Co-Founder of Dyad Medical. “This new milestone indicates the FDA’s trust in Dyad Medical’s work and enables broader adoption of imaging AI as an integral part of value-based care.”Coronary artery disease, the most common type of heart disease, is the leading cause of death, disability, and escalating healthcare costs worldwide. It is frequently treated by inserting metal stents in the hearts’ arteries. The stents help hold open the artery, resolving obstructions and blockages that impede coronary blood flow. While drug-eluting stents hinder restenosis, or future blockages, there is room to improve existing treatment options. To optimize stent design, sensitive in vivo, or within the heart, assessments are needed for preclinical and clinical evaluations. IVOCT is the only imaging method with the resolution and contrast able to accomplish this.
Until now, IVOCT imaging has not been widely adopted. It requires operators to manually interpret and analyze cardiac images, taking in a large amount of data in real-time during percutaneous coronary intervention, a minimally invasive procedure used to open clogged coronary arteries. LibbyTM IAAA provides an automated solution in cardiac image analysis and interpretation.“Stent failure has been linked to inadequate stent expansion, incomplete stent coverage of diseased segments, and untreated dissections at the stent edges,” said Dr. Shalev. “By combining AI and technology innovations, LibbyTM IAAA is able to facilitate thorough stent analysis that will help physicians and researchers make accurate observations and conclusions about overall patient health.”A leading research hospital has already signed on to use LibbyTM IAAA. Dyad Medical is a member of QualComm’s XR Enterprise Program, the NVIDIA Inception Program, and the MassChallenge Boston Accelerator Program. To learn more about Dyad Medical’s automated medical image analysis products, visit http://www.dyadmed.com.








